A palatable choice for 10 years
When Krka started the journey to developing our Milprazon range, one of the key goals was making palatability a key feature to aid administration.
Launched in 2015, Milprazon (milbemycin oxime/praziquantel) is now a well-established dog and cat-worming solution in the UK and covers a wide range of indications including heartworm, lungworm, eyeworm and intestinal worms including tapeworm.
In 2022 Krka further upgraded the range to the highly palatable Milprazon CHEWABLE.

Milprazon CHEWABLE remains the only wormer in its category with proven palatability for dogs based on EMA Testing Guidelines that are referenced in the SmPC. In a study1, more than 85% of dogs ate the tablet ‘voluntarily’, with 75% taking it unprompted from their food bowl.
How did we do it?
When pet risk factors and lifestyle indicate regular worming is needed either quarterly or monthly, research has proved palatability is a critical element in tablet formulation to increase acceptance in dogs. In general, more complex palatants that have appealing taste, smell, and mouth feel enhance voluntary uptake.2
The Milprazon CHEWABLE range uses natural pig liver flavouring to provide a high level of palatability with an associated reduction of allergy concerns compared to more common meat derivatives such as beef.3
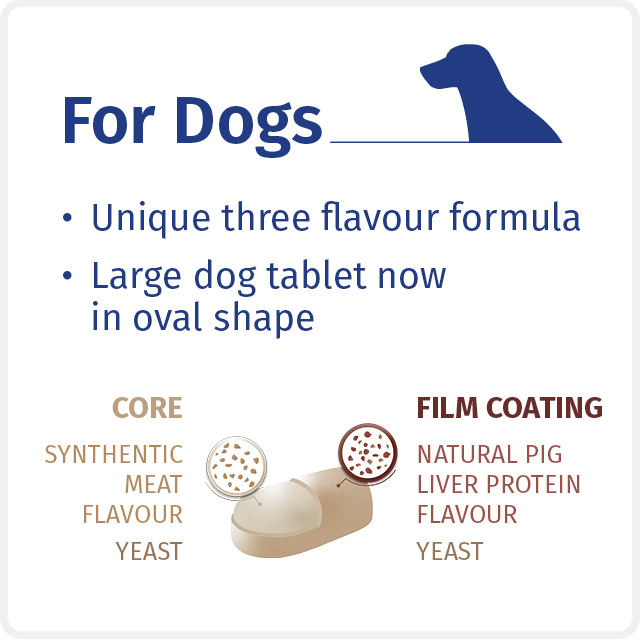
The small tablet sizes across the range combines the best features of both chewable and film-coated tablets. Research has shown in fact dosage forms that are too chewy may not be freely consumed even with complex palatants.2

For cats, small tablet size can be a real advantage providing the option that for more challenging cats it can still be administered manually without the need to break the tablet up.4 For the cat formulation Krka attained an Easy to Give award from International Cat Care.
Krka continues to innovate in the animal medicine category with new generic formulations, offering product advantages and an expanding range of therapeutic areas covered.
To find out more about the Milprazon CHEWABLE range click here.
Risk based approach assessment for prescribing parasiticides
To support the renewed focus on the use of pet lifestyle and risk-based factors for prescribing parasiticides for companion animals, Krka’s Vet2Vet Insights guide helps practices navigate this topic in an informed and effective way. Learn more here.
References:
- Cvejic D. Evaluation of the palatability of the investigational veterinary product Milbemycin oxime/ Praziquantel 12.5 mg/125.0 mg chewable tablets in dogs under field conditions [internal study number 03452-001-1-1]. München: KLIFOVET AG. 2020
- Aleo, M., Ross, S. et al. (2018) Palatability Testing of Oral Chewables in Veterinary Medicine for Dogs. Open Journal of Veterinary Medicine, 8, 107-118.
- Mueller et al. Critically appraised topic on adverse food reactions of companion animals (2): common food allergen sources in dogs and cats; BMC Veterinary Research (2016) 12:9 DOI 10.1186/s12917-016-0633-8
- Sivén, M., Savolainen, S., Räntilä, S., Männikkö, S., Vainionpää, M., Airaksinen, S., Raekallio, M., Vainio, O. and Juppo, A.M. (2017), Difficulties in administration of oral medication formulations to pet cats: an e-survey of cat owners. Veterinary Record, 180: 250-250. https://doi.org/10.1136/vr.103991





